|
|
A multicentre, randomised, phase II trial of brigatinib consolidation versus observation or durvalumab in patients with unresectable stage III NSCLC and ALK-rearrangement, after definitive chemo-radiotherapy.
BOUNCE: Brigatinib post definitive chemo-radiotherapy in patients with ALK-fusion non-small cell lung cancer
Trial Scheme
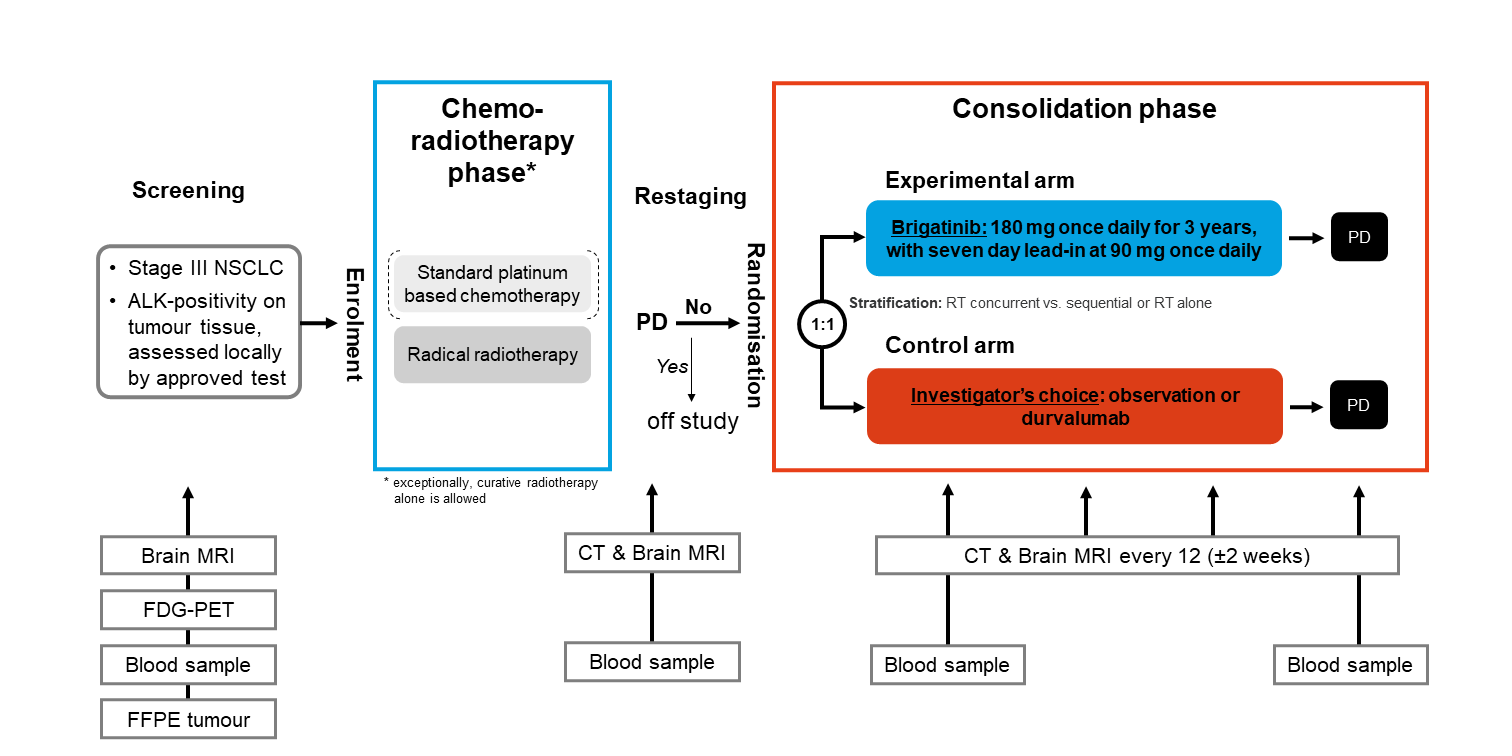
| Primary Endpoint: | Progression-free survival (PFS) according to RECIST v1.1 |
| Secondary Endpoints: |
Overall survival CNS-relapse-free survival Pattern of disease progression Toxicity according to CTCAE v5.0 |
| Target Sample Size: | 44 randomised patients (approximately 55 enrolled) |
| Protocol Release Date: | 6 February 2024 |
Trial Organisation |
|
|
Trial Chair: |
Rafal Dziadziuszko, Gdansk, Poland |
| Trial Co-Chair: |
Sanjay Popat, London, United Kingdom |
| Sponsor: | ETOP IBCSG Partners Foundation |
| Coordinating Group: | ETOP IBCSG Partners Foundation |
| Participating Groups: | SLCGT |
| Participating Countries: |
France, Italy, Poland, Spain, and the United Kingdom |
| Registrations: |
EU CT number: 2022-502467-38 clinicaltrials.gov: NCT05718297 |
Contact
Rico Hunkeler (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


