|
|
A multicentre, single-arm phase II trial of adagrasib in patients with KRASG12C-mutant NSCLC, including the elderly (≥70 years) or patients with poor performance status.
ADEPPT: Adagrasib in patients with KRASG12C-mutant NSCLC who are elderly or have poor performance status
The aim of the trial is to assess the clinical efficacy of adagrasib treatment, in terms of objective response, in patients with KRASG12C‑mutant NSCLC, including the elderly (≥70 years) or patients with poor performance status (ECOG PS=2).
Trial Scheme
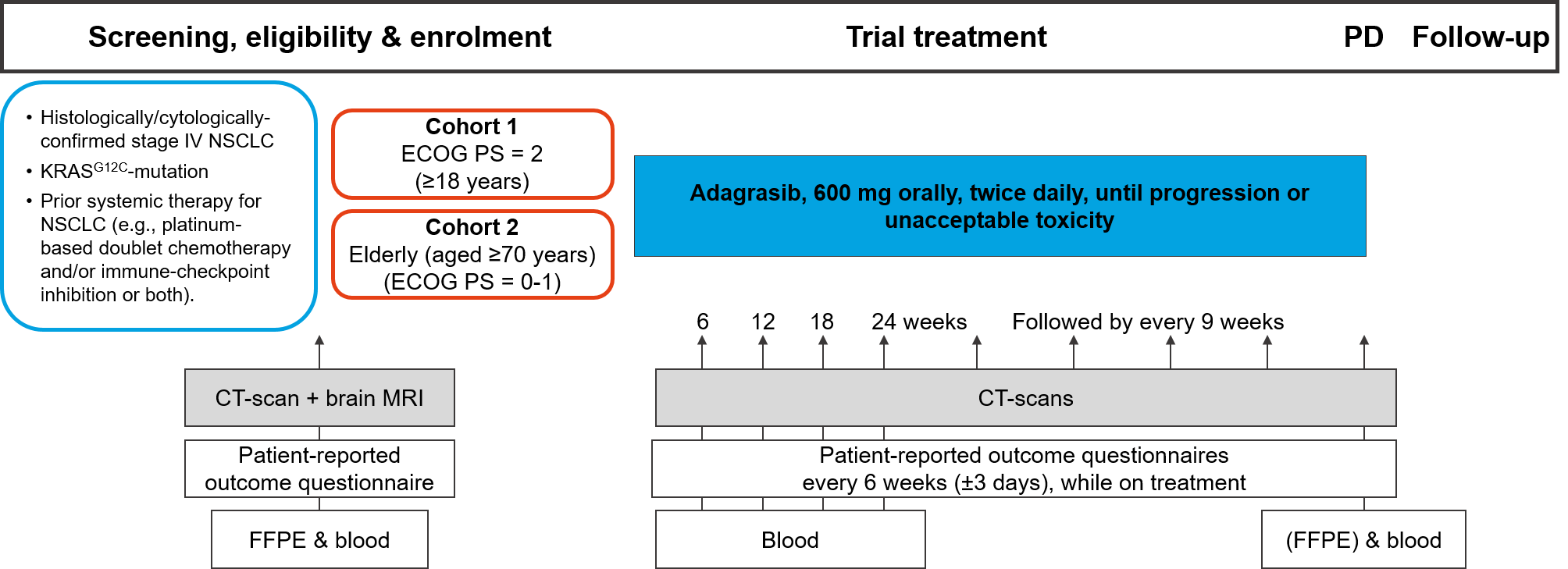
| Primary Endpoint: | Objective Response Rate (ORR) per RECIST v1.1, assessed at 12 weeks |
| Secondary Endpoints: |
Durable clinical benefit Time to progression Progression-free Survival Overall Survival Safety Patient-related outcomes |
| Target Sample Size: | 68 enrolled patients |
| Protocol Release Date: | 15 July 2022 |
Trial Organisation |
|
| Trial Chair: | Jarushka Naidoo, Dublin, Ireland |
| Trial Co-Chairs: |
Colin Lindsay, Manchester, United Kingdom Bartomeu Massuti, Alicante, Spain |
| Sponsor: | ETOP IBCSG Partners Foundation |
| Coordinating Group: |
ETOP IBCSG Partners Foundation |
| Participating Groups: |
Cancer Trials Ireland (CTI) and the Spanish Lung Cancer Group (SLCG) |
| Participating Countries: |
Belgium, France, Ireland, Italy, Spain, and United Kingdom |
| Registrations: |
EudraCT number: 2022-002736-31 clinicaltrials.gov: NCT05673187 |
Contact
Virginia Rodriguez Martinez (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


