|
|
A multicentre single-arm phase II trial assessing the safety and efficacy of first-line osimertinib and locally ablative radiotherapy in patients with synchronous oligo-metastatic EGFR-mutant non-small cell lung cancer.
STEREO: Osimertinib and locally ablative radiotherapy in patients with synchronous oligo-metastatic EGFR mutant NSCLC
The trial explores the safety and efficacy of osimertinib treatment and locally ablative radiotherapy to all cancer sites, in patients with synchronous oligo-metastatic EGFR-mutant NSCLC.
Trial Scheme
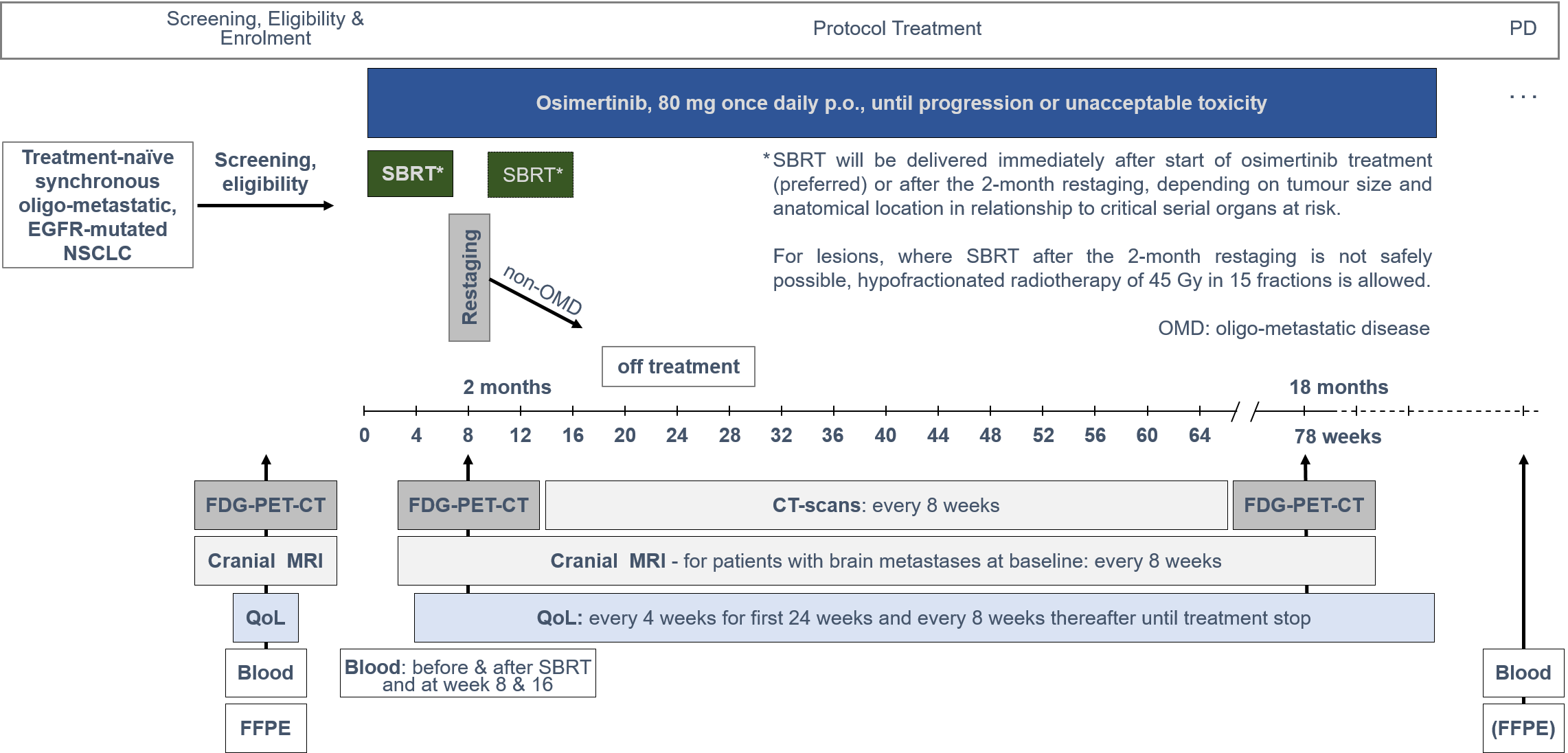
| Primary Endpoint: | Rate of grade ≥2 pneumonitis, requiring medical treatment, observed any time during the first 18 months of follow-up from enrolment, in the primary-endpoint safety cohort. If safety is proven, efficacy will be hierarchically tested in terms of PFS according to RECIST v1.1, in the efficacy cohort. |
| Secondary Endpoints: |
Overall survival Pattern of disease progression Distant progression-free survival Objective response rate Duration of response Toxicity by CTCAE v5.0 Symptom-specific and global quality of life |
| Target Sample Size: | 60 enrolled patients |
| Protocol Release Date: | 26 April 2020 |
| Trial Activation Date: | 19 November 2021 |
Trial Organisation |
|
| Trial Chair: | Matthias Guckenberger, Zürich, Switzerland |
| Trial Co-Chairs: |
Egbert F. Smit, Amsterdam, the Netherlands Cho Byoung Chul, Seoul, South Korea |
| Sponsor: | ETOP IBCSG Partners |
| Coordinating Group: | ETOP IBCSG Partners |
| Participating Group: | SLCG |
| Participating Countries: |
Europe: Italy, the Netherlands, Poland, Spain, Sweden, and Switzerland Asia: Singapore and South Korea |
| Registrations: |
EudraCT number: 2020-004114-35 clinicaltrials.gov: NCT04908956 |
Contact
Julien Orgül (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


