|
|
A single arm phase II trial evaluating the activity of alectinib for the treatment of pretreated RET-rearranged advanced NSCLC
ALERT-lung: ALEctinib for the treatment of pretreated RET-rearranged advanced non-small cell lung cancer
The RET gene encodes for a transmembrane receptor with tyrosine kinase activity. It is involved in cell proliferation, migration, differentiation, and in neuronal navigation. RET rearrangements occur in 1%-2% of patients with adenocarcinoma. Alectinib is a highly selective next generation ALK inhibitor that has demonstrated potent anti-tumour activity in RET rearranged NSCLC in preclinical studies and early phase trials.
The aim of this trial is to evaluate the activity of alectinib as second-line treatment of pre-treated RET-rearranged advanced NSCLC.
Trial Scheme
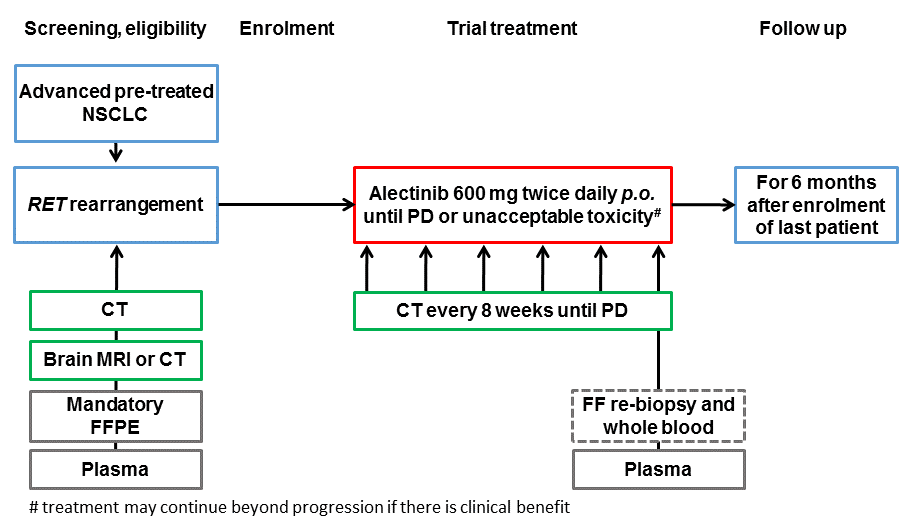
| Primary Endpoint: | Best overall response (OR = CR or PR), per investigator assessment |
| Secondary Endpoints: | Best overall response per independent review Disease control at 24-weeks Progression-free survival Overall survival |
| Target Sample Size: | 44 patients |
| Final Accrual: | 14 patients |
| Protocol Release Date: | 28 September 2017 |
| Trial Activation Date: | 18 April 2018 |
| First Patient In: | 06 November 2018 |
| Accrual Closure Date: | 03 March 2021 |
| Global Trial Completion Date: |
03 March 2021 |
Trial Organisation |
|
| Trial Chairs: | Enriqueta Felip and Jürgen Wolf |
| Trial Co-Chair: | Egbert F. Smit |
| Sponsor: | ETOP IBCSG Partners |
| Coordinating Groups: | ETOP IBCSG Partners in collaboration with Lung Cancer Group Cologne |
| Participating Groups: | Cancer Trials Ireland, SAKK and SLCG |
| Participating Countries: | Belgium, France, Ireland, Italy, Germany, Netherlands, Portugal, Slovenia, Spain, and Switzerland |
| Registrations: |
EudraCT number: 2017-002063-17 ClinicalTrials.gov: NCT03445000 |
Contact:
Virginia Rodriguez Martinez (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


