|
|
A randomised phase II trial of osimertinib and bevacizumab versus osimertinib alone as second-line treatment in stage IIIb-IVb NSCLC with confirmed EGFRm and T790M
BOOSTER: Osimertinib and Bevacizumab versus Osimertinib alOne as Second-line Treatment in stage IIIb-IVb NSCLC with confirmed EGFRm and T790M
First-generation EGFR tyrosine kinase inhibitors provide a significant clinical benefit for patients with advanced EGFR-mutant NSCLC. However, most patients ultimately develop disease progression, driven by the acquisition of a second T790M EGFR TKI resistance mutation.
Osimertinib (AZD9291) is an irreversible third-generation tyrosine kinase inhibitor of both EGFRm-sensitizing and T790M resistance mutants.
Bevacizumab is a humanized antibody targeting the vascular endothelial growth factor VEGF, which plays an important role in tumour angiogenesis.
A combination treatment that target both tumour cells and tumour microenvironment (such as angiogenesis) may be a promising strategy for further improving efficacy outcomes in patients with EGFRm (exon 19 deletion or exon 21 L858R) NSCLC following progression on EGFR TKI therapy and other lines of therapy.
The aim of the BOOSTER trial is to assess the efficacy of osimertinib plus bevacizumab versus osimertinib alone for patients with locally advanced or metastatic (stage IIIb-IVb) EGFRm NSCLC with a T790M resistance mutation at progression on prior EGFR TKI therapy.
Trial Scheme
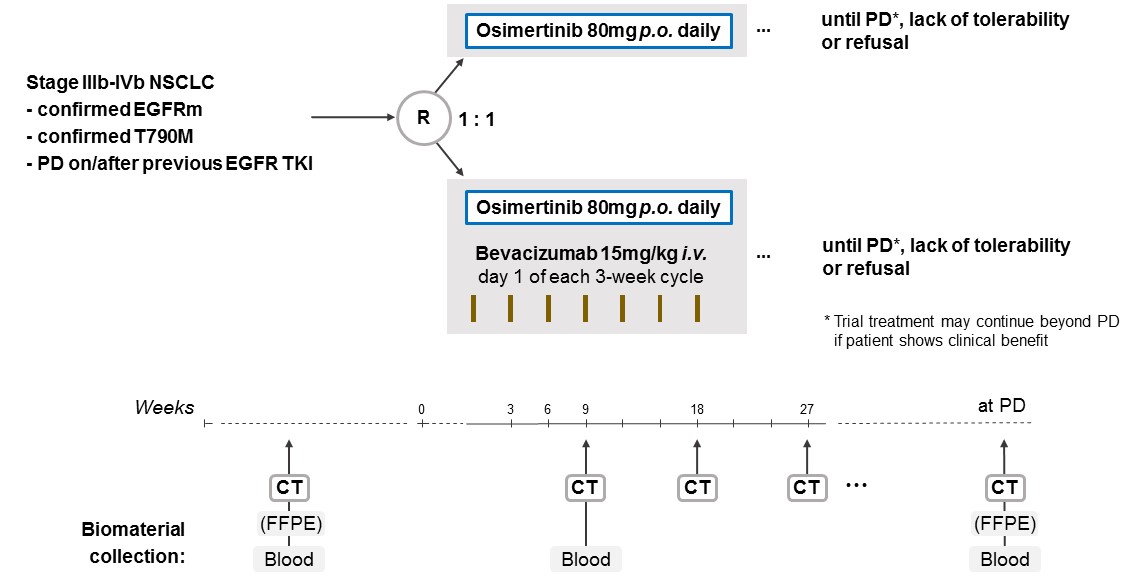
| Primary Endpoint: | Progression-free survival | ||
| Secondary Endpoints: |
Objective response Disease control Overall survival Adverse events |
||
| Target Sample Size: | 154 Patients | ||
| Final Accrual: | 155 Patients | ||
| Protocol Release Date: | 13 December 2016 | ||
| Trial Activation Date: | 05 April 2017 | ||
| First Patient In: |
31 May 2017 | ||
| Accrual Closure Date: | 21 February 2019 | ||
| Global Trial Completion Date: | - | ||
Trial Organisation |
|||
| Trial Chairs: | Solange Peters and Rolf Stahel | ||
| Trial Co-Chairs: | Ross Soo and Ji-Youn Han | ||
| Sponsor: | ETOP IBCSG Partners |
||
| Coordinating Group: | ETOP IBCSG Partners | ||
| Participating Groups: | Cancer Trials Ireland, SAKK and SLCG | ||
| Participating Countries: |
Ireland, Netherlands, Spain, Switzerland, South Korea, and Singapore | ||
| Registrations: |
EudraCT number: 2016-002029-12 ClinicalTrials.gov: NCT03133546 |
||
Contact
Julien Orgül (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


