A single-arm phase II trial of the addition of niraparib to anti-PD-L1 antibody maintenance in patients with SLFN11-positive, extensive-disease small cell lung cancer.
Niraparib added to anti-PD-L1 antibody maintenance in SLFN11-positive, extensive-disease SCLC
An international, multicentre, single-arm phase II trial in patients with extensive-disease small cell lung cancer and high SLFN11-expression who have not progressed during first-line standard chemo-immunotherapy and are planned for maintenance therapy with immune-checkpoint inhibition .
Trial Scheme
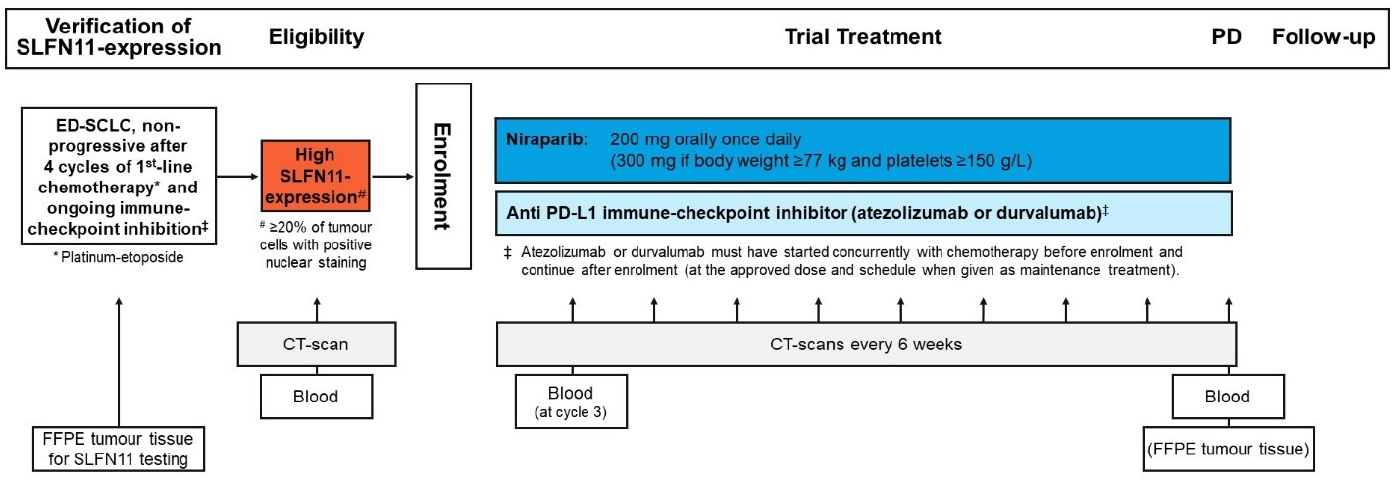
| Primary Endpoint: | Progression-free survival (PFS) rate at 3 months by investigator assessment (according to RECIST v1.1) |
| Secondary Endpoints: |
Progression-free survival (PFS) Overall survival (OS) Disease control rate (DCR) by investigator assessment (according to RECIST v1.1) Adverse events according to CTCAE v5.0 |
| Target Sample Size: | 44 enrolled patients |
| Protocol Release Date: | 21 February 2023 |
Trial Organisation |
|
| Trial Chair: |
Markus Joerger, St. Gallen, Switzerland |
| Trial Co-Chair: |
Antonio Passaro, Milano, Italy |
| Sponsor: |
ETOP IBCSG Partners Foundation |
| Coordinating Group: |
ETOP IBCSG Partners Foundation |
| Participating Groups: | SAKK and SLCG |
| Participating Countries: |
France, Italy, Romania, Switzerland and Spain |
| Registrations: |
EU CT number: 2022-502092-33 clinicaltrials.gov: NCT05718323 |
Contact
Virginia Rodriguez Martinez (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


