|
|
A multicentre single-arm phase II trial of amivantamab, lazertinib plus bevacizumab in patients with EGFR-mutant advanced NSCLC with progression on previous third-generation EGFR-TKI.
AMAZE-lung: Amivantamab, lazertinib and bevacizumab in patients with EGFR-mutant advanced non-small cell lung cancer with progression on previous third-generation EGFR-TKI
The AMAZE-lung study aims to test the efficacy of amivantamab, bevacizumab and lazertinib in patients with EGFR-mutant advanced NSCLC.
Trial Scheme
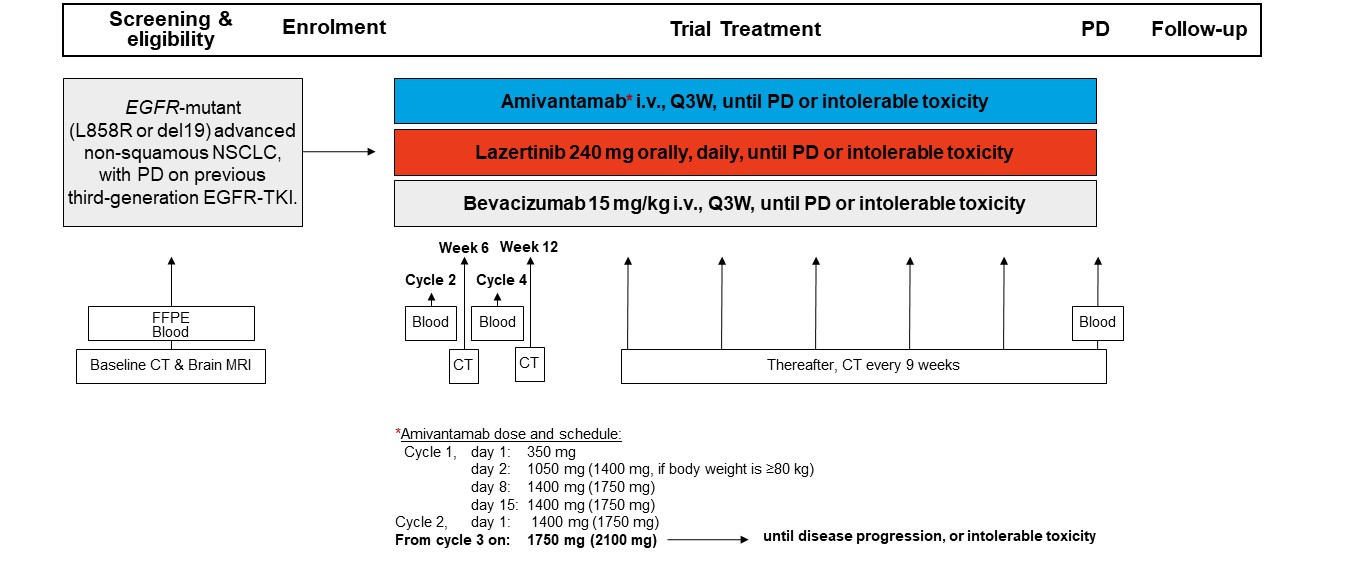
| Primary Endpoint: | Objective response rate (ORR), investigator assessed, at 12 weeks according to RECIST v1.1 |
| Secondary Endpoints: |
Duration of response (DoR) Progression-free survival (PFS) according to RECIST v1.1 Disease control rate (DCR) according to RECIST v1.1 Overall survival (OS) Safety and tolerability (CTCAE v5.0) |
| Target Sample Size: | 60 enrolled patients |
| Protocol Release Date: | 14 September 2022 |
Trial Organisation |
|
| Trial Chair: | Ross Soo, Singapore |
| Trial Co-Chair: |
Sanjay Popat, London, United Kingdom |
| Sponsor: | ETOP IBCSG Partners Foundation |
| Coordinating Group: | ETOP IBCSG Partners Foundation |
| Participating Groups: | Spanish Lung Cancer Group (SLCG) |
| Participating Countries: |
France, Italy, the Netherlands, Singapore, South Korea, Spain, Switzerland, United Kingdom |
| Registrations: |
EudraCT number: 2021-002337-42 clinicaltrials.gov: NCT05601973 |
Contact
Uli Kodjadjiku (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


