Link to EORTC-8111-LCG
A randomised, open-label phase III trial evaluating the addition of denosumab to standard first-line anticancer treatment in advanced NSCLC
SPLENDOUR: Survival imProvement in Lung cancEr Induced by DenOsUmab theRapy
Denosumab is a monoclonal antibody targeting and inhibiting RANKL, a protein that acts as the primary signal for bone resorption. The trial was designed to investigate the potential of denosumab added to standard treatment (chemotherapy) compared to standard treatment alone to increase survival of patients with advanced NSCLC with or without bone metastasis in advanced unselected treatment-naïve patients.
After the excellent initial recruitment, the accrual rate has dramatically slowed down, potentially due to more attractive treatment alternatives for this patient population, in the context of a competitive landscape of ongoing immune-oncology clinical trials. The trial Steering Committee has therefore decided to close the recruitment prematurely. No safety concerns led to this decision. Treatment and follow-up of all included patients continues as specified in the protocol. A manuscript with the final analysis is currently in preparation.
Trial Scheme
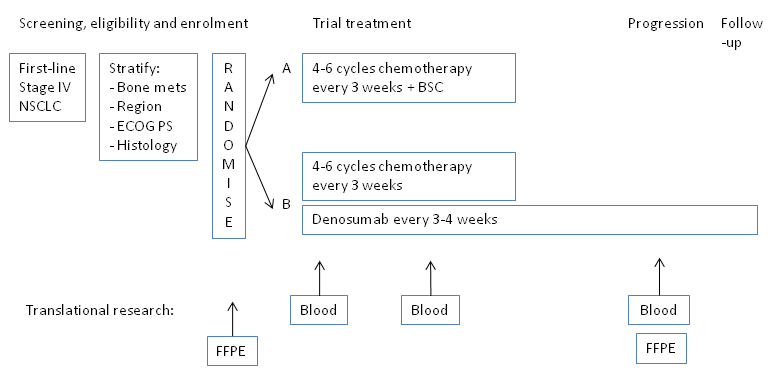
| Primary Endpoint: | Overall survival |
| Secondary Endpoints: |
Progression free survival Objective response Toxicities of treatment Evaluation of potential predictive biomarkers for denosumab activity |
| Target Sample Size: | 1000 Patients |
| Final Accrual: | 514 Patients |
| Protocol Release Date: | 27 May 2014 |
| Trial Activation Date: | 18 August 2014 |
| First Patient In: | 12 January 2015 |
| Accrual Closure Date: | 10 January 2018 |
| Global Trial Completion Date: | 10 September 2018 |
Trial Organisation |
|
| ETOP Trial Chair: |
Solange Peters |
| Sponsor: |
ETOP IBCSG Partners |
| Coordinating Group: | EORTC |
| Participating Countries: |
Austria, Belgium, France, Germany, Ireland, Italy, Slovenia, Spain, Switzerland, and United Kingdom |
| Registrations: |
EudraCT number: 2013-003156-21 ClinicalTrials.gov: NCT02129699 |
Contact
Uli Kodjadjiku (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


