|
|
A randomised open-label phase II trial of consolidation with nivolumab and ipilimumab in limited-stage SCLC after chemo-radiotherapy
STIMULI: Small cell lung carcinoma Trial with nivolumab and Ipili-MUmab in LImited disease
Chemo-radiotherapy is the current standard treatment approach in limited-stage (stage I-IIIB) small cell lung carcinoma (SCLC) with a median survival of 16 to 24 month and only 15 – 25% long-term survivors.
Nivolumab and ipilimumab are humanized monoclonal antibody that target the immune checkpoint receptors PD-1 and CTLA-4, respectively and inhibit the interaction with their ligands.
The aim of the trial is to evaluate whether nivolumab plus ipilimumab consolidation treatment after completion of standard chemo-radiotherapy and prophylactic cranial irradiation is superior in terms of overall survival and progression-free survival compared to standard chemo-radiotherapy and prophylactic cranial irradiation alone.
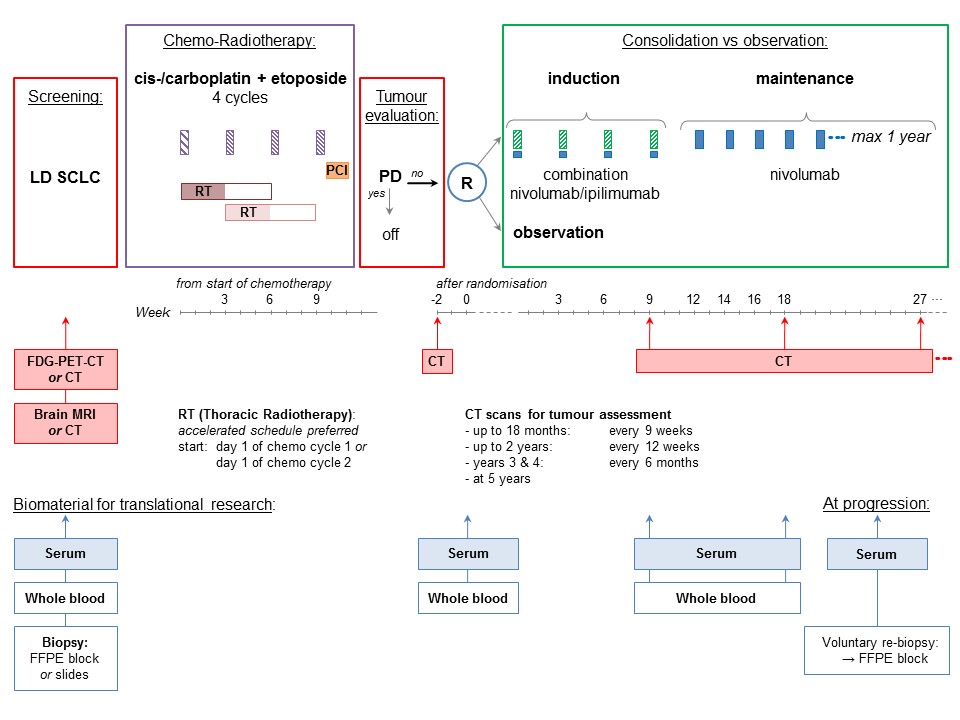
Trial Scheme
Trial Treatment
Patients who are randomised to the experimental arm will receive nivolumab 1mg/kg and ipilimumab 3 mg/kg in the induction phase, followed by 240mg nivolumab as maintenance treatment for 1 year. A maximum of one cycle of platinum based chemotherapy can be administered before enrolment. In this case radiotherapy should optimally start concomitantly with chemotherapy cycle 2 and no later than cycle 3.
| Primary Endpoints: | Progression free survival and Overall survival |
| Secondary Endpoints: |
Objective response Time to treatment failure Toxicities of treatment |
| Target Sample Size: | 260 Randomized Patients |
| Final Accrual: | 174 Randomized Patients |
| Protocol Release Date: | December 2013 / AMD1 September 2015 |
| Trial Activation Date: | 25 April 2014 |
| First Patient In: | 28 July 2014 |
| Accrual Closure Date: | 30 April 2019 |
Trial Organisation |
|
| Trial Chairs: | Solange Peters and Dirk De Ruysscher |
| Sponsor: | ETOP IBCSG Partners |
| Coordinating Groups: | ETOP IBCSG Partners in collaboration with IFCT |
| Participating Groups: | ALTG/CTC, IFCT, SAKK and SLCG |
| Participating Countries: | Belgium, France, Germany, Netherlands, Spain, Switzerland, United Kingdom, Australia, and New Zealand |
| Registrations: |
EudraCT number: 2013-002609-78 ClinicalTrials.gov: NCT02046733 |
Contact
Julien Orgül (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland