|
|
An open-label phase II trial of erlotinib and bevacizumab in patients with advanced non-small cell lung cancer and activating EGFR mutations
BELIEF: Bevacizumab and ErLotinib In EGFR mut+ NSCLC
The BELIEF trial was designed to assess the long-term outcome of patients with advanced non-squamous NSCLC with activating EGFR mutations (L858R and exon 19 deletion) with or without T790M resistance mutation at diagnosis and treated with the combination of erlotinib and bevacizumab.
The final accrual goal of 109 was reached in October 2014 and the results of the primary analysis were published in Lancet Respiratory Medicine 2017; 5 (5): 435-444. Treatment and follow-up are still ongoing.
Trial Scheme
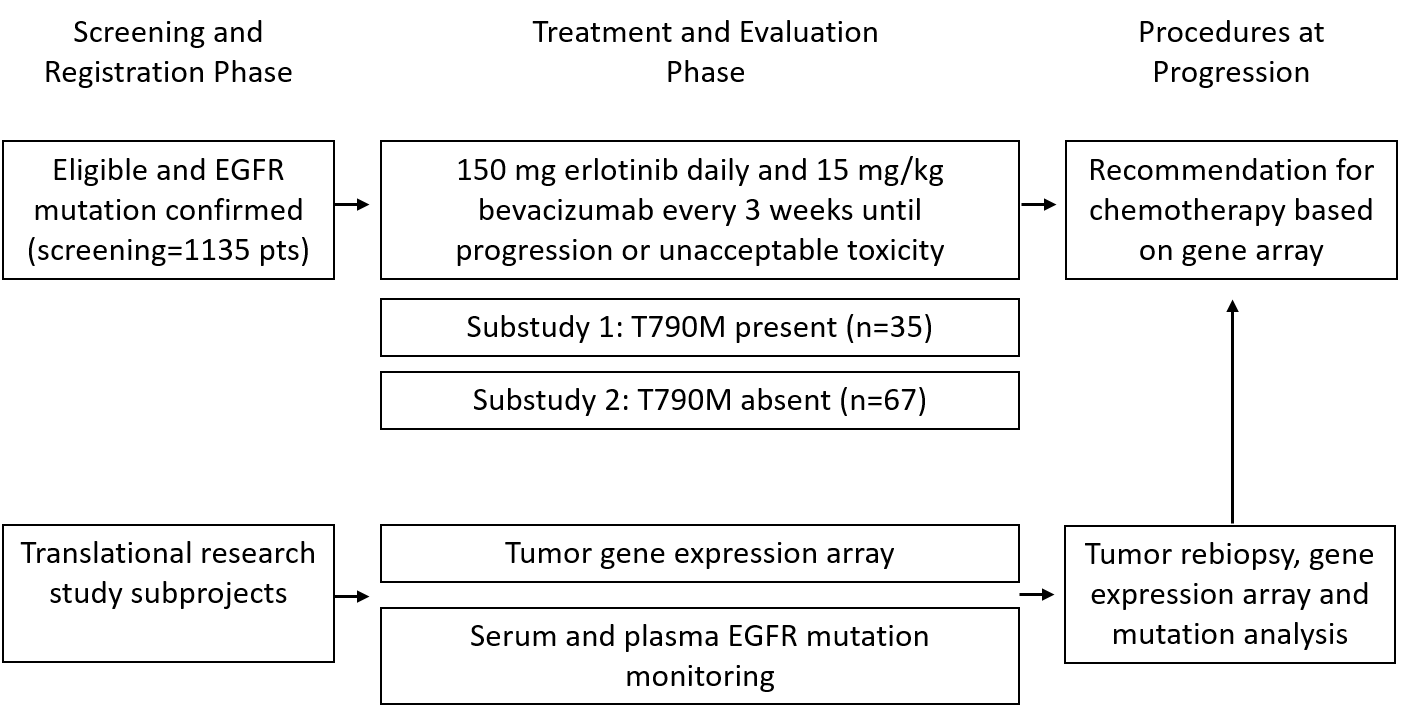
| Primary Endpoint: | Progression free survival |
| Secondary Endpoints: |
Overall survival Time to treatment failure Objective response rate Disease control rate Duration of response |
| Sample Size: | 102 Patients |
| Final Accrual: | 109 Patients |
| Protocol Release Date: | January 2012 / AMD1 November 2013 |
| Trial Activation Date: | 27 April 2012 |
| First Patient In: | 11 June 2012 |
| Accrual Closure Date: | 28 January 2015 |
| Global Trial Completion Date: | 19 October 2018 |
Trial Organisation |
|
| Trial Chair: | Rafael Rosell |
| Trial Co-Chair: | Rolf Stahel |
| Sponsor: | ETOP |
| Coordinating Groups: | ETOP in collaboration with SLCG |
| Participating Groups: | HORG, ICORG, SAKK and SLCG |
| Participating Countries: |
France, Germany, Greece, Ireland, Italy, Spain, Switzerland and United Kingdom |
| Registrations: |
EudraCT number: 2011-004481-15 ClinicalTrials.gov: NCT01562028 |
Contact
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


