|
|
A multicentre randomised open-label phase III study of stereotactic radiosurgery, in addition to standard systemic therapy for patients with metastatic melanoma or newly diagnosed metastatic NSCLC and asymptomatic or oligo-symptomatic brain metastases.
USZ-STRIKE: Immunotherapy or targeted therapy with or without stereotactic radiosurgery for patients with brain metastases from melanoma or non-small cell lung cancer
The trial aims to assess the efficacy of standard systemic treatment plus stereotactic radiosurgery in patients with newly diagnosed brain metastases from melanoma or NSCLC.
Trial Scheme
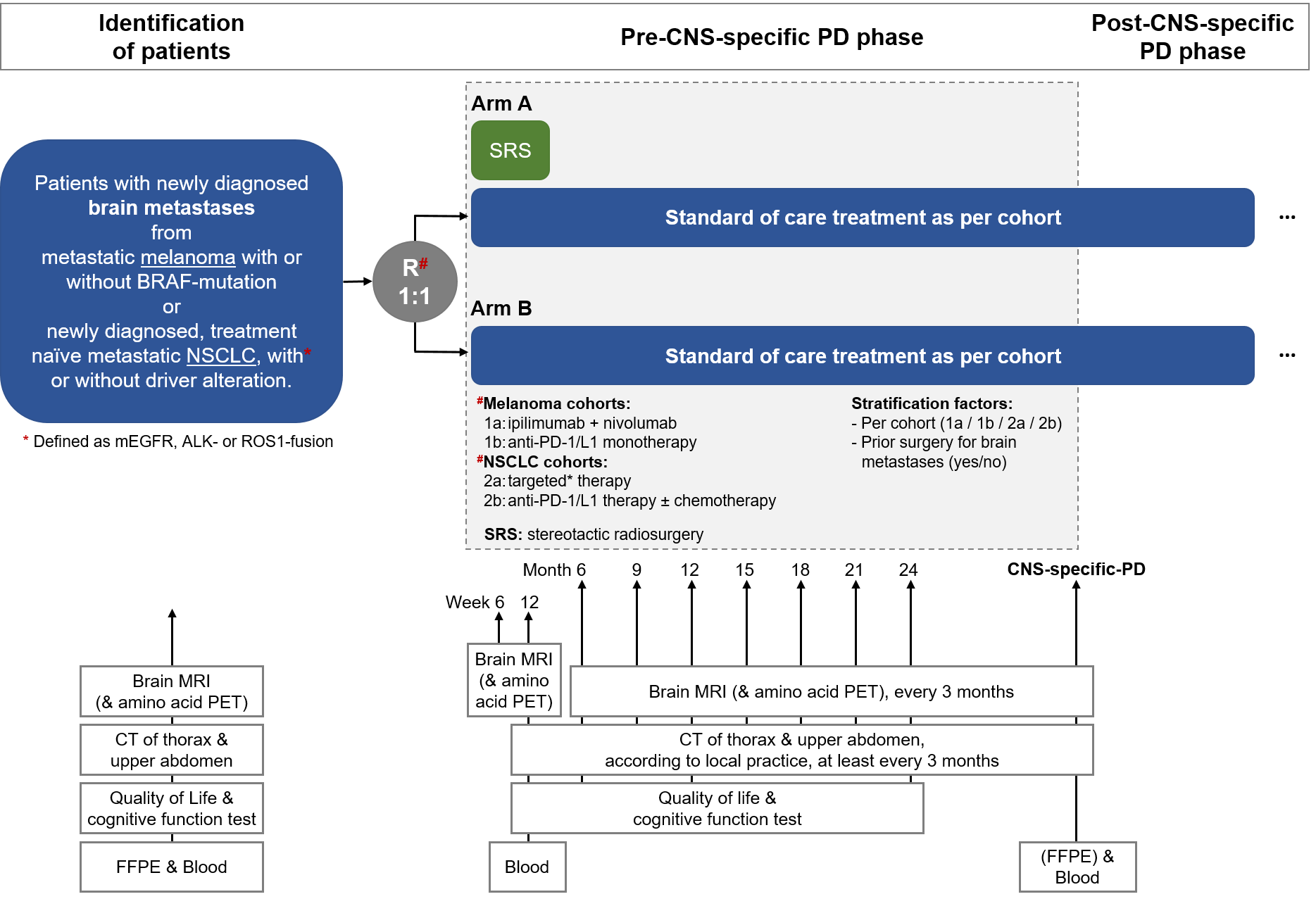
| Primary Endpoint: | CNS-specific PFS, locally assessed as per iRANO criteria |
| Secondary Endpoints: |
CNS-specific PFS per tumour cohort, locally assessed as per iRANO criteria CNS-specific PFS, overall and per tumour cohort, centrally assessed as per iRANO criteria Objective CNS-response rate, centrally assessed as per iRANO criteria Duration of CNS-response Pattern of CNS-specific progression (local versus distant progression) Extra-CNS progression, locally assessed as per RECIST v1.1 Incidence of radio-necrosis and pseudo-progression in the CNS OS, overall and per tumour cohort Neurocognitive function Quality of life and functional independence Toxicity by CTCAE v5 |
| Target Sample Size: | 190 randomised patients |
| Protocol Release Date: | 14 February 2022 |
Trial Organisation |
|
| Trial Chair: | Michael Weller |
| Trial Co-Chairs: | Rolf Stahel |
| Sponsor: | ETOP IBCSG Partners Foundation |
| Coordinating Group: | ETOP IBCSG Partners Foundation |
| Participating Countries: |
Italy, the Netherlands, Switzerland, and United Kingdom |
| Registrations: | NCT05522660 |
Contact
Julien Orgül (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


