|
|
A feasibility trial evaluating the addition of anti-PD-1 nivolumab to standard first-line chemotherapy and radiotherapy in locally advanced stage IIIA/B Non-Small Cell Lung Carcinoma
NICOLAS: NIvolumab COnsolidation with standard first-line chemotherapy and radiotherapy in Locally Advanced Stage IIIA/B Non-Small Cell Lung Carcinoma
Concomitant chemotherapy and radiotherapy is the first treatment choice for most patients with stage III non-small-cell lung carcinoma (NSCLC). However, only about 30% of patients are alive 5 years after concomitant therapy and there is an unmet need to identify new treatment options in order to improve the prognosis of patients diagnosed with locally advanced NSCLC. Based on the good results in advanced disease, immunotherapy provides a very promising approach. The monoclonal antibody nivolumab is an immune-checkpoint inhibitor that prevents the interaction between the PD-1 receptor and its ligands.
The aim of the NICOLAS trial is to assess the safety and efficacy of nivolumab when administered concurrently to standard first-line chemo-radiotherapy in locally advanced stage IIIA/B NSCLC.
Trial Scheme
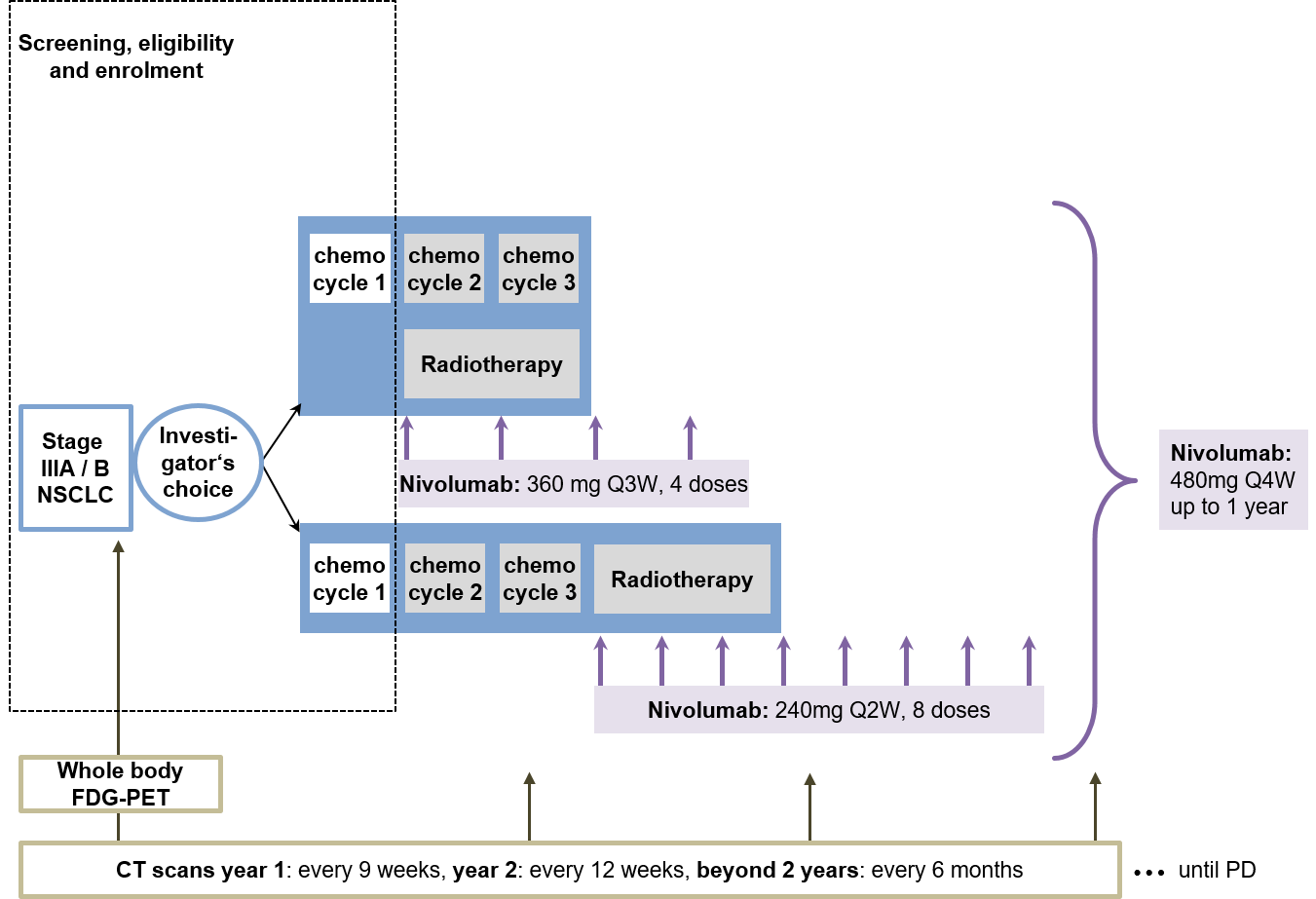
| Primary Endpoint: | Grade > 3 pneumonitis |
| Key-secondary Endpoint: |
1-Year progression-free survival |
| Secondary endpoints: |
Time to first pneumonitis Objective response rate Time to treatment failure Overall survival Toxicities of treatment |
| Target Sample Size: | 94 Patients |
| Final Accrual: |
94 Patients |
| Protocol Release Date: | August 2015 / AMD1 July 2016 / AMD2 July 2017 |
| Trial Activation Date: | 24 July 2015 |
| First Patient In: | 25 November 2015 |
| Accrual Closure Date: | 13 Februray 2019 |
| Global Trial Completion Date: | 13 February 2020 |
Trial Organisation |
|
| Trial Chairs: | Solange Peters and Dirk De Ruysscher |
| Sponsor: | ETOP IBCSG Partners |
| Coordinating Groups: | ETOP IBCSG Partners |
| Participating Countries: |
Belgium, Germany, Netherlands, Spain, and Switzerland |
| Registrations: |
EudraCT number: 2014-005097-11 ClinicalTrials.gov: NCT02434081 |
Contact
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


