|
|
A multicentre single arm phase II trial assessing the efficacy of immunotherapy, chemotherapy and stereotactic radiotherapy to metastases followed by definitive surgery or radiotherapy to the primary tumour, in patients with synchronous oligo-metastatic non-small cell lung cancer
CHESS: Immunotherapy, Chemotherapy, Radiotherapy and Surgery for Synchronous Oligo-metastatic NSCLC
Immunotherapy has substantially improved the outcome in metastatic NSCLC and has become first-line treatment of choice in patients with high PD-L1 expression and without EGFR or ALK mutations. However, only about one-third of NSCLC patients present a PD-L1 expression of ≥50%, excluding most patients from first-line immunotherapy. Various strategies are currently pursued to increase the number of patients that may benefit from immunotherapy and to further improve the outcome of patients with metastatic NSCLC.
The CHESS trial builds on encouraging data from combined chemo-radiotherapy with immune-checkpoint inhibitors and the excellent local control provided by radiotherapy and surgery.
Trial Scheme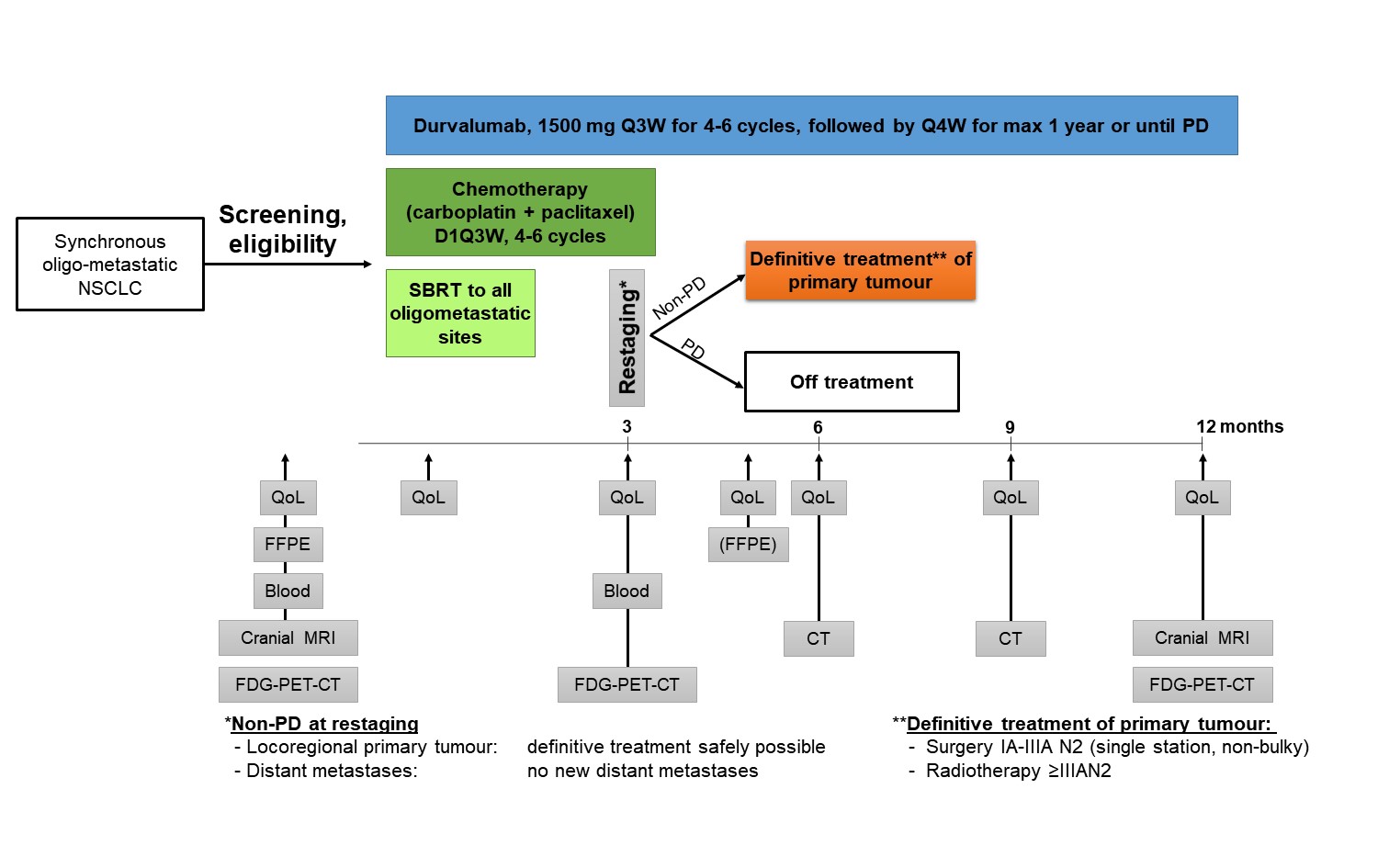
| Primary Endpoint: | Progression-free survival at 12 months |
| Secondary Endpoints: |
Overall survival Pattern of disease progression Distant progression-free survival Response to induction therapy Overall response Duration of response Toxicity before and after surgery/radiotherapy Symptom-specific and global quality of life |
| Target Sample Size: | 47 patients |
| Protocol Release Date: | 25 April 2019 |
| Trial Activation Date: | 05 September 2019 |
| First Patient In: | 19 November 2019 |
Trial Organisation |
|
| Trial Chair: | Matthias Guckenberger, Zürich, Switzerland |
| Trial Co-Chairs: |
Isabelle Schmitt-Opitz, Zürich, Switzerland Alessandra Curioni-Fontecedro, Fribourg, Switzerland |
| Sponsor: | ETOP IBCSG Partners Foundation |
| Coordinating Group: | ETOP IBCSG Partners Foundation |
| Participating Groups: | SAKK and SLCG |
| Participating Countries: |
Italy, The Netherlands, Spain, and Switzerland |
| Registrations: |
EudraCT number: 2018-003011-22 ClinicalTrials.gov: NCT03965468 |
Contact
Julien Orgül (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


