|
|
A multicentre randomised phase III trial comparing pembrolizumab versus standard chemotherapy for advanced pre-treated malignant pleural mesothelioma
PROMISE-meso: PembROlizuMab Immunotherapy versus Standard chemotherapy for advanced prE-treated malignant pleural mesothelioma
Systemic chemotherapy is currently the international standard care of treatment for patients with advanced pleural mesothelioma, where surgery is not possible. However, the median survival for these patients is only 9-12 month and there is unmet need for an effective therapy for patients with relapsed pleural mesothelioma.
Pembrolizumab is a potent and highly selective antibody, designed to directly block the interaction between PD-1 and its ligands.
The aim of the PROMISE-meso trial is to investigate whether treatment with pembrolizumab improves progression-free survival (PFS) compared to standard chemotherapy (gemcitabine or vinorelbine).
Trial Scheme
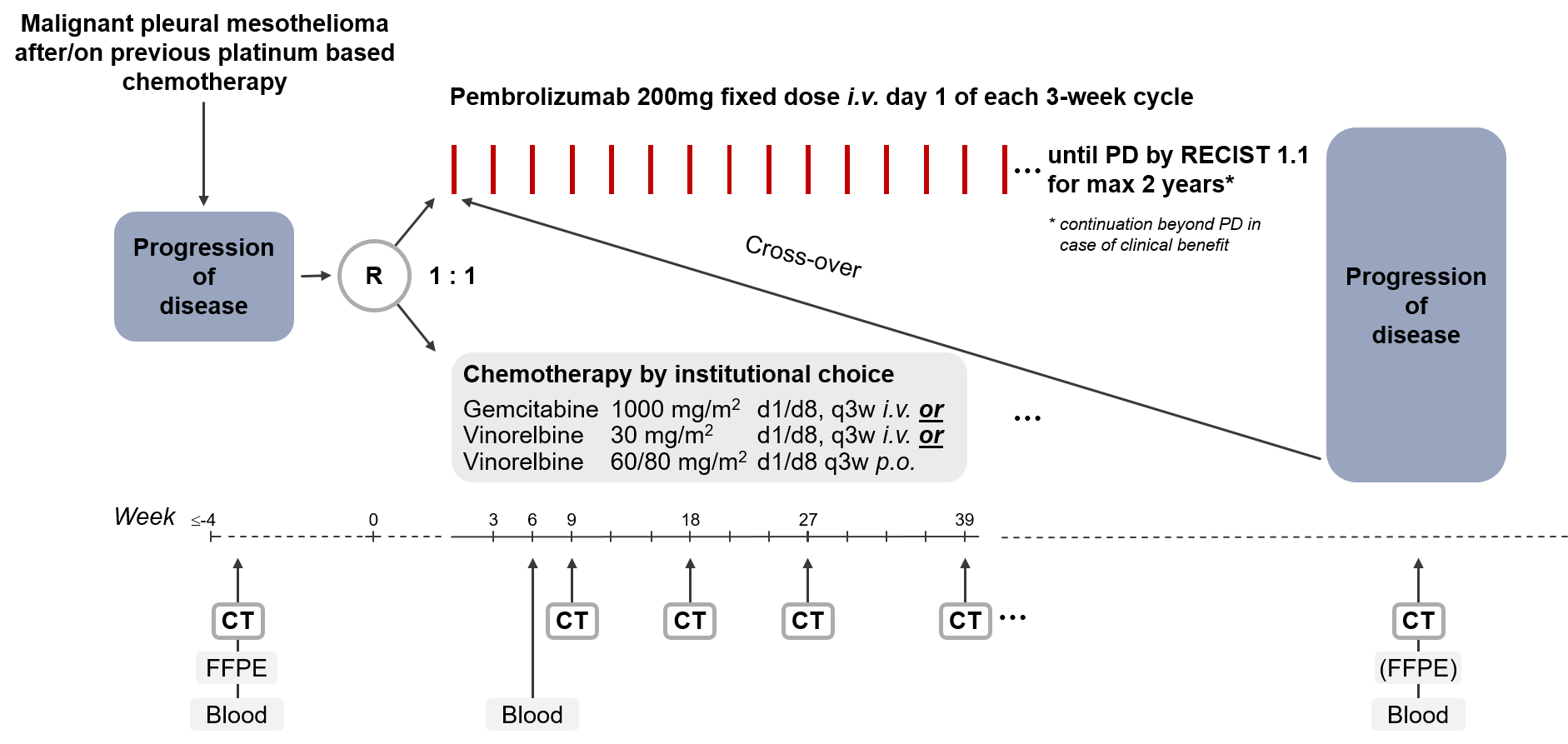
| Primary Endpoint: | Progression-free survival based on independent review |
| Secondary Endpoints: |
Objective response Overall survival Time to treatment failure Investigator assessed progression-free survival Tolerability of treatment |
| Target Sample Size: | 142 Patients |
| Final Accrual: | 144 Patients |
| Protocol Release Date: | 15 November 2016 |
| Trial Activation Date: | 02 May 2017 |
| First Patient In: | 12 September 2017 |
| Accrual Closure Date: | 14 August 2018 |
| Global Trial Completion Date: | 29 November 2021 |
Trial Organisation |
|
| Trial Chairs: | Sanjay Popat and Alessandra Curioni-Fontecedro |
| Sponsor: | ETOP IBCSG Partners |
| Coordinating Group: | ETOP IBCSG Partners |
| Participating Groups: | SAKK and SLCG |
| Participating Countries: |
Spain, Switzerland, and United Kingdom |
| Registrations: |
EudraCT number: 2016-002062-31 ClinicalTrials.gov: NCT02991482 |
Contact
Uli Kodjadjiku (Clinical Trial Manager)
ETOP IBCSG Partners Foundation
Effingerstrasse 33
3008 Bern, Switzerland


